CLINICAL EFFECTS of Ixodes holocyclus in dogs
The effects of attachment of a paralysis tick include:
Allergic reactions (mainly applies to
humans)
Foreign body granulomas (mainly humans)
Transmission of zoonoses (mainly humans)
Paralysis and the poisoning syndrome. (all
warm blooded animals)
Late effects (ie after recovery from
paralysis, seen in some dogs)
Post-mortem pathology (described in dogs)
Experimental data (Ilkiw and Turner, 1987)
Allergic reactions
Allergic reactions to ticks may be i) small local reactions, ii) large local reactions, iii)systemic reactions (anaphylaxis), and iv) unusual allergic reactions (Sonenshine, 1991).
Anaphylaxis (allergic shock) has been reported in humans following contact with Ixodes ticks. The result is collapse and prostration. Apparently many people have experienced spectacular reactions when they have come into contact with both live and dead tick products. Having a tick simply walk over a person's hand produces in some people an intense discomfort and itching. It is not yet known what particular components of the tick body cause these allergic and hypersenstive effects. It could be a water soluble component that is excreted through the cuticular canals (Jones,1991) (see toxicology).
Larvae and nymphs, as well as adults are capable of causing very dramatic allergic reactions. Dramatic local erythema (redness) and oedema (fluid swelling) may develop within 2-3 hours of attachment of even one larva. In southeast Queensland a "maddening rash" known as "scrub itch" is caused by infestation by many tick larvae- this especially affects people clearing leafy bushland such as lantana bushes. Frequently a tick embedded over an eyelid will result in gross facial and neck swelling within three hours. The person can go on to develop very severe signs of tracheopharyngeal compression within 5-6 hoursafter the first onset of symptoms. See ticks on humans for pictures and more information on allergic reactions.
Foreign body granulomas
This is recognised as a problem in some humans. In the case of attachment by an adult female it is usual for a lump to remain at the site of attachment for many weeks or even months after removal of the tick. These lumps will remain as vague irritations some 3-5 mm in diameter at the site of attachment for up to 9 months. Changes in the regional lymph nodes draining the area of attachment can also lead to the formation of chronically sensitive enlargement within the node lasting for several years, particularly those around the head and neck. It is not known whether this is due to retention of parts of the hypostome or fragments of the pedipalps or some other agent. If the hypostome is examined with a hand lens after removal of the embedded tick it is always [NF: often, ie not always] damaged. Dogs often have a lump at the site of tick attachment but this rarely seems to cause any irritation.
Zoonosis transmission
The Australian paralysis tick is able to transmit to humans the causative agents of Q Fever (Coxiella burnetii) Rickettsial Spotted Fevers (Queensland Tick Typhus (QTT), Rickettsia australis; and Flinders Island Spotted Fever (FISF), R. honei) and probably a Lyme-like disease (Borrelia sp). It is possible that the domestic dog and cat may occasionally afflicted by similar diseases [although I have not, however, seen documented evidence of this]. In fact there is so far no firm documentation of any disease being transmitted by the paralysis tick to dogs [NF]. In Northern Queensland the Brown Dog Tick (Rhipicephalus sanguineus), is apparently responsible for transmitting the Babesia canis organism causing anaemia in young dogs. Ehrlichia canis, also transmitted by R. sanguineus, is still regarded as an exotic parasitic blood disease in Northern Australia, but it may already be established there (Greening et al, 1995).
See also Tick-Transmitted Diseases of humans (Australia) and Tick-Transmitted Diseases of Dogs (Australia)
Paralysis and the poisoning syndrome (in dogs and cats)
Effects on specific body systems- molecular pathophysiology, locomotor, oropharyngeal, demeanour, respiratory, urinary, ocular, gastro-oesophageal, cardiovascular, neuroendocrine, metabolic.
Overall presentation
Whilst most cases of paralysis are associated with Ixodes holocyclus, paralysis in Australia has also been reported in association with Ixodes cornuatus and Ixodes hirsti. Ixodes holocyclus has been reported to produce paralysis in many species (see hosts) including dogs, cats, sheep, calves, foals, pigs, llamas, chickens and humans (principally infants). The disease usually ocurs after infestation with adult female ticks, although heavy infestations with nymphs or larvae are capable of causing paralysis. The following discussion relates mainly to the toxic effects in dogs and cats.
It is believed that the physical effects seen in tick paralysis are caused by various neurotoxins produced in the salivary glands of engorging ticks. Several toxic fractions have been isolated from the salivary glands of Ixodes holocyclus, including a protein neurotoxin (named holocyclotoxin) that causes paralysis, and another toxin that is lethal but non-paralysing. See toxicology.
A single engorged adult female tick can kill the largest dog. Clinical signs of tick intoxication are usually observed 3-7 days after attachment of the tick. However the period preceeding the onset of symptoms appears to depend on the rate of engorgement of the parasites rather than on a fixed time period. This period may be prolonged up to 2 weeks in cool weather. Even with massive infestations, signs do not appear until at least the third day. The shortest interval of 3 days might occur with multiple ticks and in warm weather. In some dogs, clinical signs may not be seen until all ticks have engorged and dropped off replete. From experience gained in deliberate loading of kennelled dogs for antivenom production, one tick can remain half engorged on a dog for 7-8 days before eventually engorging. A tick can also remain attached and partially engorged for up to three weeks before either completing its engorgement and dropping off or dropping off without repletion. Thus it is cited in some studies that for some susceptible dogs up to 4-8 ticks are required to induce paralysis.
Although an alteration in the quality of the dog's bark can often be appreciated early in the course of the disease, the first consistent sign is a slight wobbliness of the hindquarters that rapidly progresses so that in a few hours the dog is unable to stand. The motor paralysis rapidly ascends to involve the forelimbs so that the animal lies in lateral recumbency. Body temperature usually is normal at first, but animals can become hypothermic as the disease progresses. The pupils are widely dilated and eventually fail to respond to light [this has not been a consistent finding for me]. Regurgitation or vomiting often occurs, sometimes as the first sign of tick intoxication, and may persist throughout the course of the disease. Respiration becomes slow and laboured, with a prolonged expiratory phase. A grunting respiratory noise is often present (resulting from closure of vocal cords during early expiration). Dyspnoea becomes increasingly apparent and is accompanied by cyanosis and coarse crackles on auscultation as animals approach death. Untreated dogs (without pre-existing immunity) usually die within 24-48 hours of the onset of obvious clinical signs.
Similar signs are seen in cats with tick paralysis; affected animals are typically distressed and agitated. Initially there is often a change in voice, especially noticeable in Burmese and Siamese cats, which may be accompanied by retching or vomiting. Pupillary dilation is prominent in cats. Vomiting is rare in comparison to the dog.
Some dogs with tick intoxication do not present with a straightforward ascending flaccid motor paralysis. Instead they may present because of anorexia, intractable vomiting or loss of voice in the absence of significant appendicular weakness. It is unclear whether true vomiting or regurgitation occurs in these cases. Other dogs are presented for lethargy, inappetance, coughing, gagging, or groaning. However, there is good evidence of laryngeal paresis, pharyngeal dysfunction and megaoesophagus in some reports. Vomiting may result from a central action of tick toxin on the chemoreceptor region of the medulla, whereas regurgitation is probably referable to a megaoesophagus. Localised mainfestations of tick paralysis, such as asymmetrical facial paralysis and anisocoria have been reported in dogs and children. Exposure keratoconjunctivitis may result from eyelid paralysis. The different manifestations of tick paralysis may result from variations in the susceptibility of the host and the number and virulence of the ticks. A rapid onset of signs appears to be associated with more severe disease and a higher likelihood of fatality
Effects on specific body systems
Molecular Pathophysiology
The tick toxin is believed to cause a flood of Ca++ into cells. The myofibrils of certain cardiac and smooth muscles appear to be paralysed in a contracted state. The myofibrils of acetylcholine vesicles at the neuromuscular junction may interfere with Ach release and hence paralysis of skeletal muscles, but these remain in a relaxed state (Atwell RB, 2003).
Locomotor
Detailed neurologic testing demonstrates reduced muscle tone and diminished to absent myotatic reflexes early in the course of the disease [I have found some early cases to be normo- or even hyper-reflexic; one case in a young Golden Retriever actually showed a moderately clonic patellar reflex- this is normally a finding in chronic neuropathies- in this case two old tick craters were found and the clinical signs of hindlimb weakness remained static for about a week until finally a third tick was removed and the more typical hypotonia developed]. Although withdrawal reflexes are initially normal they become progressively slower and weaker as the condition progresses. Although proprioception and cutaneous sensation are generally thought to be preserved, diminished perception of noxious tactile stimuli occasionally has been noted in experimental studies.
Oropharyngeal
The gag reflex is consistently depressed, and the inability to swallow results in drooling of saliva. When the pharynx is palpated it feels relatively vacuous, because it is lacking tone, and pooling of saliva can be pronounced. Reflux oesophagitis resulting from megaoesophagus can be expected to augment this salivation.
Demeanour
Anxiety, apprehension and stress in an animal developing tick paralysis may have several causes. There may be "frustration" with not being able to ambulate normally. Dyspnoea and the need to make slow deep forced expirations would also be expected to reduce tolerance to stress. Accumulation of saliva within the dilated paralysed pharynx may lead to gagging and a choking sensation, possibly even laryngospasm (although laryngeal dysfunction should counteract this). An inablitiy to cough may likewise cause a sense of dyspnoea. A reduced capacity to pant and therefore thermoregulate could cause hyperthermic stress. Vomiting and regurgitation may further increase the threat of respiratory distress. Some cats are particularly susceptible to the stress of handling and may rapidly develop cyanosis. Psychological stress is minimised by handling the animal as little as possible, keeping the environment relatively cool (but not cold so as to be shivering or hypothermic), reducing strong lighting, minimising noise, and reducing the threat of unfamiliar people and animals. The use of a tranquilliser such as acepromazine often reduces apparent psychlogical stress (as an alpha blocker it may also be beneficial in reducing hypertension and hence pulmonary congestion/oedema). Judicious use of atropine may be beneficial in cases where excessive saliva is interfering with respiration either directly by physical means or indirectly by causing laryngospasm. Hypertension may however be exacerbated by atropine and so concurrent use of acepromazine would seem logical.
Cardiovascular
In uncomplicated cases the colour of the mucous membranes is a fairly normal pink. The capillary refill time is normal or even more rapid than usual. The femoral pulse is normal or relatively strong and slow. The original reseach of Ilkiw demonstrated that most dogs developed hypertension, which was relatively mild, and more systolic than diastolic. It was postulated that this was related to increased sympathetic tone, particularly stimulation of alpha receptors. Even if this is the mechanism of hypertension it is not known whether it is a direct effect of tick toxins or a reflex response. Nor is it known which of the 7 types of alpha receptors is involved, nor to what degree, nor which tissues (eg central or peripheral, venous or arterial, pulmonary or systemic etc) (Atwell and Fitzgerald, 1994).
ECG effects. In Ilkiw and Turner's experiments (1988) the ECG changes which occured were not consistent from dog to dog nor from stage to stage (see data table below). If an arrhythmia occurred in Stage 1 it tended to be a tachycardia, whereas in Stages 2 and 3 a brady- or tachycardia was present, and in Stages 4 and 5 a bradycardia predominated. Changes in potassium and calcium, if they occurred, were not sufficiently great to explain any ECG changes. Myocardial hypoxia in stages 4 and 5 could have explained some of the abnormalities, especially in the later stages- for example S-T segment depression, T wave abnormalities, sinus tachycardia, premature ventricular contractions, ventricular tachycardia and bradycardia. In the earlier stages, before hypoxaemia came significant, it was postulated that autonomic dysfunction in the form of excessive sympathetic activity or excessive parasympathetic was responsible for ECG abnormalities.
The finding of "Long QT Syndrome" may explain sudden deaths in dogs both during obvious paralysis and also after apparent recovery (Campbell and Atwell, 2002). Features of the syndrome in man are: delayed cardiac repolarisation, alteration in ion flux, polymorphic ventricular tachycardia (Torsade de pointes) and precipitated by sympathetic stimuli. Dogs seem to show similar features. It is suggested that the risk factors of stress, exercise and exposure to drugs known to prolong the QT interval (eg Cisapride) are minimised. The period of risk appears to extend well beyond the recovery from paralysis. Beta blockers theoretically may reduce the risk caused by sympathetic stimulation.
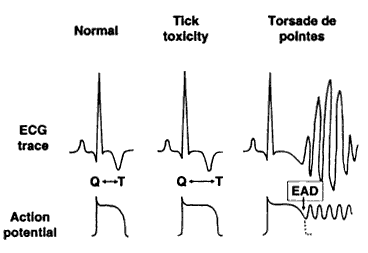
Long QT Syndrome: Campbell and Atwell, 2002
Effects on cardiac contractility in dogs. There is reduced diastolic function - ie a failure of myocardium to relax sufficiently for filling. This effect diminishes after 2 days as the toxin detaches. This is accompanied by pulmonary oedema (crackles) with an associated restrictive dyspnoea pattern (i.e. shallow polypnoea, at least initially). At this time there is an elevation in PCV (> 0.50 L/L) whilst protein remains normal. No myocardial structural changes have been found under electron microscopy (Atwell RB, 2003).
Vascular beds. In dogs there are significant regional arterio-venous anastomoses that can be 'paralysed' in contraction. This can affect thermoregulation and promote hypothermia (Atwell RB, 2003).
Respiratory
There is a progressive fall in respiratory rate and plasma
bicarbonate with a rise in expiratory time. From Stage 2 a fall
in arterial oxygen tension develops and the alveolar-arterial
oxygen tension difference rises [indicating that V/Q mismatch or
O2 diffusion are more limiting than ventilation]. Minute
respiratory volume also falls significantly by Stage 4. This is
due mainly to the decline in respiratory rate because the
expiratory (~tidal) volume does not fall (at least not in the
first 4 Stages; Stage 5 not having been measured).
Ilkiw and Turner (1987b) relate how the "grunting"
respiration is similar to that observed in babies with pulmonary
congestion and oedema where the vocal cords are closed during
expiration to asist re-expansion of collapsed portions of the
lungs. The "grunting sound" is caused by the sudden
release of the vocal cords after a prolonged expiratory phase
against closed vocal cords.
Because of the partial paralysis of the respiratory muscles the
accessory muscles are activated to contribute to respiratory
effort.
Overall, respiratory compromise may result for a number of
reasons- hypostatic congestion, pulmonary oedema (hypertension
plus an unknown factor), aspiration of pharyngeal or gastric
secretions, neuromuscular paralysis of respiratory muscles, and a
hypothesised centrally mediated respiratory depression. It has
also been suggested that the expiratory dyspnoea and grunt
represent a respiratory obstructive pattern. Rather than "splinting
the alveoli", this breathing pattern may be dilating
constricted small airways- the sudden opening of the glottis
allowing a prolonged expiratory phase whilst the glottic closure
is permitting a compromise to occur between the effort to do so
and the benefit of easier expiratory flow (Atwell and
Fitgerald, 1994).
The respiratory pattern in dogs seems to differ from that in humans. In man it appears that as the disease progresses respiration becomes fast and shallow (Cleland, 1912; Eaton, 1913; Ferguson, 1924; Hamilton, 1940 and Pearn, 1966). The type of respiration in man following tick envenomation is more consistent with that observed in diseases such as poliomyelitis, where there is partial or complete paralysis of the muscles of respiration. In these cases respiratory respiratory rate increases whilst tidal volume decreases and the accessory muscles contribute to respiration (Hobes, 1955; Taylor, 1955). It has therefore been suggested that factors other than than neuromuscular paralysis affect respiration- eg pulmonary congestion and oedema and central respiratory depression (Ilkiw and Turner, 1987b).
Note how in Stage 1 the vocal cords are effective in completely closing and preventing expiratory flow in the middle part of the exhalation. The small peaks would correspond with the end-expiratory grunts. In contrast, in Stage 3 they are only partly effective, probably because there is paralysis of the vocal cords themselves.
As mentioned above, this stage is thought to represent an obstructive breathing pattern (i.e. slow deep dyspnoea) caused by bronchial spasm. The characteristic grunting is thought to be associated with release of glottic closure. The glottic closure probably represents an attempt to increase airway pressure as a splinting mechanism for the narrowed bronchi. A double expiratory effort may also have a diaphragmatic component, although diaphragmatic fatigue is also suspected (Atwell, 2003).
Urinary
Some male dogs have been observed to have difficulty emptying their bladder when recovering from paralysis; their bladders are difficult to express and may require catheterisation to be emptied- possible reflex dyssynergia- ie incoordination between voluntary striated muscle urethral sphincter and involuntary smooth muscle sphincter (personal observation)]
Ocular
Pupils are often dilated in cases of tick paralysis. In cats this may be an early sign. It could be caused by either a direct paralytic effect on iris sphincter muscle or part of the general effect of increased sympathetic tone (stress). A tick in the vicinity of an eyelid may cause a localised facial palsy resulting in exposure keratoconjunctivitis. A resulting corneal ulcer might result in uveitis and hence a small pupil in the paralysed eye (miosis). The eyelid paralysis may last for several weeks beyond the course of the systemic disease.
Oesophageal
Megoesophagus (MO) has been reported in association with tick paralysis (Malik, King and Allen, 1988). Given that most of the canine oesophagus has a striated musculature, such an effect is not unexpected. Fitzgerald (1998) has found that most dogs exhibiting gagging/retching of white froth have evidence of megaoesophagus on plain lateral chest radiographs. A recent study (Campbell and Atwell, 2001) found that 70% of dogs presented for tick poisoning showed radiographic evidence of megaoesophagus. Megaoesophagus was graded by comparing the maximum width of the of the dilated oesophagus with the length of the third thoracic vertebra. Gradings were either absent, minor (< T3 length) or marked (> T3 length). Whilst 48% (n=22) showed clinical signs consistent with MO, these were not associated with the radiographic appearance of MO. Furthermore, the presence or degree of MO based on radiographs taken on patient admission was not related to the gait or respiratory scores or to reduced or absent gag reflex.
Laryngeal
The dysphonia associated with early stages of tick paralysis in dogs and cats is attributed to paralysis of intrinsic laryngeal muscles. However, the end-expiratory grunt heard with advancing tick paralysis is thought to reflect a compensatory closure of the glottis that may increase end-expiratory pressure and thereby "splint" the alveoli against collapse. In the most severe cases this mechanism may be lost resulting in alveolar hypoventilation and consequent ventilation/perfusion mismatching (shunting).
Neuro-endocrine
It is thought that there is an increase in sympathetic tone. This may be a direct effect of tick toxins or an indirect effect of stress. Having an increased sympathetic tone is consistent with the hypertension and dilated pupils seen but less consistent with the relatively slow pulse, the normal colour and refill time of the mucous membranes and the increased saliva production. In defence of the theory of a generalised increase in sympathetic tone: The slower heart rate could be normal reflex bradycardia resulting from the hypertension. The colour and refill of the mucous membranes could be normal despite increased sympathetic tone because vascular beds other than mucosae may be constricted and so contributing to the increase in vascular resistance. Increased saliva production could be an overriding response to loss of pharyngeal tone, of megaoesophagus or of gastro-oesophageal reflux.
Metabolic
Overall, from the work of Ilkiw et al (1987a), there are minimal changes in serum biochemistry (and haemotology) until an advanced stage of the disease is reached. The parameters measured were Na, K, Ca, Mg, PO4, Total Protein, Albumin, Total CO2, Urea, Cholesterol, Glucose, Alkaline Phosphatase, Total Bilirubin and Creatine Phosphokinase. The results of this research found the following changes:
During Stage 1 protein rose significantly from control. The other measurements did not change significantly.
During Stage 2 potassium, albumin, urea and total bilirubin fell significantly, while glucose and cholesterol rose significantly above control values. There was no significant difference from control in the other measurements.
At Stage 3 there were significant falls in potassium, total carbon dioxide, urea and total bilirubin, while glucose and cholesterol were significantly elevated from control. The other measurements did not change significantly.
At Stage 4 significant falls were found in potassium and urea, while glucose and cholesterol rose significantly from control. There were no significant differences for the other measurements.
At Stage 5 phosphate, cholesterol, glucose and creatine phosphokinase rose significantly, while total bilirubin fell significantly. The other measurements did not differ significantly from control.
This article had the following discussion: "It was anticipated that few biochemical values would be altered in this disease. However, since these indices have not been measured previously, it was thought that detailed investigation should be performed to establish what changes, if any, occur. Many of the significant changes in these measurements are difficult to interpret individually, but viewed together they could represent the biochemical response to sympathetic stimulation of the adrenal medulla, causing release of adrenaline and nor-adrenaline or release of adrenocorticotrophic hormone, resulting in stimulation of the adrenal cortex to secrete corticosteroids. Release of any of these hormones could cause the elevations fo glucose, cholesterol and haemoglobin, as well as the fall in potassium (Goodman and Gilman, 1975). Although phosphate showed a time variation in the control dogs, the elevation in the tick infested dogs at Stage 5 was physiologically abnormal. Severe muscle cell damage, with liberation of phosphate into the blood, could explain the elevated level (W Hensley, personal communication). Active muscle cell lysis was also suggested by the elevation in creatine phosphokinase. An elevation in creatine phosphokinase was reported in a case of tick paralysis caused by the Amercian tick Dermacentor andersoni, and it was thought that the tick toxin caused muscle damage by interference with cellular energy metabolism pathways (Boffey and Paterson, 1973)."
Hydration
Dehydration does not appear to be a major problem in the early stages of tick paralysis despite the lack of food or water intake. Maintenance requirements need to be met as the need arises although gving "sub-maintenance" fluids has been suggested (see treatment-dogs).
Late effects
Some dogs die unexpectedly with exercise (e.g. swimming) after having recovered from paralysis. Others develop arrhythmias. [NF: I have seen one young Border Collie dog which had a history of having been successfully treated for tick paralysis four times and was presented for episodes resembling syncope- this dog was auscultated with a severe cardiac rhythm disturbance but unfortunately an ECG was not performed] These cases support a possible direct cardiotoxic effect of either the tick toxins or of hypoxaemia. There has also been an observation of poor return to full capacity in working dogs following tick paralysis which may similarly reflect cardiac compromise (Atwell and Fitgerald, 1994). After recovery, a convalescent period of up to 2 weeks, with restricted exercise and avoidance of high temperatures is advised.
In the USA, Lyme disease occurs in a wide range of animals as well as in humans. There dogs, cats and horses may develop chronic disease problems following a tick bite. It is not known, however, whether a clinical borreliosis of domestic animals occurs in Australia. So far there is only some heresay evidence to support this possibility. Some animals fail to recover completely after tick bites and develop arthritis, heart disease or CNS disturbances. Such cases may respond to treatment with antibiotics (Collins, 1997). Perhaps a test will become available in the future.
Post-mortem pathology
In the experimental work of Ilkiw, Turner and Howlett (1987), in which eight crossbred dogs of unknown history were infected with 3 or 4 ticks each, the post-mortem findings of the dogs which died were as follows: "Histological examination of the tissues removed at post mortem revelaed similar changes in all dogs. In the myocardium the small blood vessels, and in some dogs the large blood vessels, appeared moderately to severely congested with slight disassociation of the muscle bundles. The liver sections showed moderate to severe acute passive venous congestion. In one dog early centrilobular necrosis was noted throughout the section. The lungs were heavy and congested and in some cases frothy fluid was present in the trachea and bronchi. Histologically there was collapse with moderate to severe generalised pulmonary congestion, and in some animals pulmonary oedema. In the kidney there was severe congestion of all blood vessels and glomerlui.". In this study 7 of the dogs developed tick paralysis and died; one dog survived without showing any clinical signs. The brain and spinal cord were not examined.
| The following table summarises the data
from the research of Ilkiw et al: clinical signs |
||||||
TABULATED SUMMARY OF ILKIW and TURNER's EXPERIMENTS |
||||||
PARAMETER |
CONTROL |
STAGE 1 paresis |
STAGE 2 unable to walk |
STAGE 3 unable to right |
STAGE 4 unable to right and lacking limb withdrawal reflexes |
STAGE 5 moribund |
| article 1 article 2 article 3 article 4 |
- n=10 n=14 n=6 |
n=7 n=4 n=5 n=5 |
n=7 n=4 n=6 n=5 |
n=7 n=4 n=3 n=4 |
n=7 n=4 n=6 n=4 |
n=7 n=5 - n=4 |
the different colours represent the different treament groups used in each of Ilkiw's papers all figures represent MEAN +/- SEM * = p<0.05, ** = p<0.02, *** = p<0.01, **** = p<0.001 |
||||||
| article 1 | Ilkiw JE and Turner DM and Howlett CR (1987) Infestation on the dog by the paralysis tick Ixodes holocyclus 1. Clinical and histological findings. Australian Veterinary Journal 64 (5) 137-139. Staging system 1-5 as defined in table below. | |||||
| article 2 | Ilkiw JE and Turner DM CR (1987a) Infestation on the dog by the paralysis tick Ixodes holocyclus 2 Blood gas and pH, haematological and biochemical findings. Australian Veterinary Journal 64 (5) 139-141. Staging system as for article 1. | |||||
| article 3 | Ilkiw JE and Turner DM CR (1987b) Infestation on
the dog by the paralysis tick Ixodes holocyclus 3
Respiratory Effects. Australian Veterinary Journal 64
(5) 142-144. Staging system as follows: Stage 1 - the dogs showed paresis when walked Stage 2 - the dogs were unable to stand but could right Stage 3 - the dogs were unable to right Stage 4 - moribund |
|||||
| article 4 | Ilkiw JE and Turner DM CR (1988)
Infestation on the dog by the paralysis tick Ixodes
holocyclus 4 Cardiovascular Effects. Australian
Veterinary Journal 65 (8) 232-235. Staging
system as follows: Stage 1 - the dogs showed ataxia when walked Stage 2 - the dogs were unable to stand but could right themselves Stage 3 - the dogs were unable to right themselves Stage 4 - within 4 hours of death Stage 5 - just prior to death |
|||||
CLINICAL SIGNS |
||||||
| control | 1 | 2 | 3 | 4 | 5 | |
paresis - The signs observed were firstly a change in character of the bark to a hoarse "husky" type and then a slight paresis of the hindlimbs. The dogs appeared alert, ate and drank normally and did not vomit. Coughing was observed in one dog. The character of respiration was normal. |
unable to walk - The dogs were in lateral recumbency, but could right themselves and hold this position if prompted. They crawled around the floor, were alert and responded by tail-wagging when approached. Withdrawal reflexes were present in all limbs when interdigital skin was pinched. There was no apparent central awareness of pain when the skin of the neck or forelimbs was pinched, but painful stimulation elsewhere resulted in movement and the dogs would look at that area. The pupils were normal in size and responsive to light. Although the gag reflex was diminished, the dogs appeared to be able to swallow, as no saliva was noticed to pool on the floor. Two dogs vomited during this stage; one vomited white frothy material, while the other vomited bile-stained material. Retching could be elicited if food was placed in front of some dogs. There was a slight change in respiratory pattern with respiration becoming more noticeable. |
unable to right - The dogs lay in lateral recumbency and despite attempts to right themselves were unable to do so. Withdrawal reflexes were usually present in all limbs, but were slower and weaker. There was no response to a painful stimulus to the neck, between the scapulae and along the forelimbs. The pupils were normal to dilated, but responsive to light. The nictitating membranes were half-way across the eyes. The gag reflex was depressed and saliva pooled on the floor in front of the dogs. Respiration was of a "grunting" type with forced expiration. |
unable to right and lacking limb withdrawal reflexes - There were spontaneous movements of all limbs, although no response was observed to painful stimulation on the neck, thorax, forelimbs, and down the hindlimbs. Painful stimulus to the abdomen or the tail caused attempts at movement. In most dogs the pupils were dilated, the pupillary light reflex was absent in some cases and the nictitating membrane more than half-way across the eye. The gag reflex was depressed and pooling of saliva on the floor in front of the dogs was marked. There appeared to be loss of bladder control with urinary incontinence. Respiration was forced and "grunting" in type. |
moribund - These dogs were within 2 h of death. All withdrawal reflexes had disappeared and the distal half of the tail was the only area which responded with movement to a painful stimulus. The gag reflex was depressed and there was marked pooling of saliva on the floor in front of the dogs. The pupils were dilated and pupillary light reflexes were absent. Respiration was forced and "grunting", the dogs appeared agitated and the limbs seemed to move with respiration. As this stage progressed, the lips were drawn back with each breath and the colour of the mucous membranes appeared grey. Respiration then became gasping and intermittent. The pupils were widely dilated and the corneas dry. |
||
| Temp C | 38.61 +/-0.16 | 38.70 +/-0.11 | 38.58 +/-0.06 | 38.38 +/-0.21 | 37.92 +/-0.21 | 37.16 +/-1.01 |
BLOOD GASES |
||||||
| CONTROL | 1 | 2 | 3 | 4 | 5 | |
PaO2 mmHg |
92.22 +/- 1.63 |
97.45 +/- 3.69 |
88.13 +/-1.64 |
89.88 +/- 2.73 |
74.47 +/-5.26 |
55.64 +/-5.997*** |
| 92.86 +/-1.18 | 78.18 +/-6.05 | 73.18 +/-4.20* | 79.07 +/-7.64 | 57.94 +/-4.53*** | ||
| 85.9 +/-2.3 | 85.9 +/-2.6 | 82.9 +/-4.2 | 80.5 +/-2.5 | 72.6 +/-4.4* | 62.4 +/-2.8** | |
| PaCO2 mmHg | 30.78 +/-0.98 | 25.40 +/- 2.45 | 31.73 +/-2.45 | 31.00 +/-1.83 | 38.84 +/-4.75* | 51.36 +/-3.35*** |
| 32.32 +/-1.03 | 31.33 +/-1.10 | 32.10 +/-2.66 | 32.63 +/-0.49 | 38.0 +/-2.42 | ||
| 33.9 +/-2.1 | 32.2 +/-2.6 | 33.3 +/-2.6 | 36.9 +/-1.7 | 36.8 +/-3.9 | 44.6 +/-1.5*** | |
pHa |
7.368 +/-0.008 |
7.395 +/-0.035 |
7.378 +/-0.010 |
7.390 +/-0.011 |
7.318 +/-0.016 |
7.203 +/-0.015*** |
| 7.389 +/-0.006 | 7.358 +/-0.018 | 7.359 +/-0.025 | 7.329 +/-0.008 | 7.314 +/-0.017* | ||
| 7.405 +/-0.004 | 7.424 +/- 0.009 | 7.395 +/-0..017 | 7.385 +/-0.009 | 7.353 +/-0.011** | 7.302 +/-0.025* | |
Std HCO3 mmol/L |
19.61 +/-0.47 |
19.20 +/- 0.87 |
20.08 +/-0.45 |
19.98 +/-0.20 |
19.75 +/-0.78 |
17.74 +/-0.54*** |
| 21.23 +/-0.60 | 19.45 +/-0.91**** | 20.35 +/-1.45 | 18.60 +/-0.85 | 19.55 +/-0.61** | ||
PAO2-PaO2 mmHg |
17.35 +/-1.03 |
33.25 +/-5.9 |
37.35 +/-1.48*** |
30.77 +/-7.82 |
45.42 +/-2.71**** |
|
OTHER RESPIRATORY PARAMETERS |
||||||
| CONTROL | 1 | 2 | 3 | 4 | 5 | |
f BPM |
29.9 +/-2.5 |
20.2 +/-1.4*** |
18.3 +/-1.9*** |
12.7 +/-0.7** |
13.3 +/-1.1*** |
|
VE mL |
213.6 +/-34.9 |
200.6 +/-53.4 |
193.4 +/-38.3 |
224.7 +/-84.4 |
199.8 +/-51.6 |
|
V'E L |
5.686 +/-0.791 |
3.912 +/-0.937 |
3.651 +/-0.554 |
2.954 +/- 1.263** |
2.556 +/-0.592*** |
|
tE sec |
1.29 +/-0.09 |
1.90 +/-0.09*** |
1.87 +/-0.28* |
2.53 +/-0.30 |
2.68 +/-0.44*** |
|
FECO2 mmHg |
15.02 +/-0.44 |
12.50 +/-0.83 |
13.80 +/-1.08 |
11.40 +/-1.40 |
16.00 +/-1.07 |
|
BIOCHEMISTRY |
||||||
| CONTROL | 1 | 2 | 3 | 4 | 5 | |
| NNa mmol/L | 146.6 +/-0.4 | 145.5 +/-1.3 | 146.3 +/-0.5 | 146.3 +/-0.6 | 144.5 +/-0.9 | 144.0 +/-2.6 |
| K mmol/L | 4.51 +/-0.07 | 4.48 +/-0.11 | 3.90 +/-0.12** | 4.08 +/-0.14 | 3.83 +/-0.13** | 4.04 +/-0.40 |
| Ca mmol/L | 2.61 +/-0.01 | 2.59 +/-0.04 | 2.53 +/-0.02 | 2.53 +/-0.08 | 2.55 +/-0.05 | 2.60 +/-0.07 |
| Mg mmol/L | 0.77 +/-0.02 | 0.75 +/-0.04 | 0.71 +/-0.02 | 0.71 +/-0.03 | 0.079 +/-0.08 | 0.92 +/-0.12 |
| PO4 mmol/L | 1.32 +/-0.04 | 1.53 +/-0.15 | 1.28 +/-0.10 | 1.43 +/-0.14 | 1.82 +/-0.11 | 2.23 +/-0.12*** |
| TP g/L | 65.5 +/-0.7 | 67.8 +/-2.0* | 66.3 +/-0.9 | 66.8 +/-1.3 | 69.8 +/-3.9 | 74.6 +/-4.8 |
| ALB g/L | 29.4 +/-0.4 | 29.5 +/-1.2 | 27.3 +/-1.0** | 28.3 +/-1.7 | 28.3 +/-1.3 | 29.8 +/-1.6 |
| TCO2 mmol/L | 20.96 +/-0.77 | 15.32 +/-1.92 | 17.18 +/-0.39 | 17.40 +/-0.73*** | 19.70 +/-0.80 | 17.86 +/-0.99 |
| U mmol/L | 6.50 +/-0.50 | 4.21 +/-0.36 | 3.57 +/-0.39* | 3.50 +/-0.46** | 4.46 +/-0.50* | 4.86 +/- 0.79 |
| CHOL mmol/L | 4.79 +/- 0.20 | 5.11 +/-0.14 | 5.61 +/-0.51 | 5.14 +/-0.24 | 6.99 +/-1.00*** | 6.85 +/-0.82* |
| GLUC mmol/L | 6.13 +/-0.14 | 6.24 +/-0.52 | 6.99 +/-0.03*** | 6.56 +/-0.24** | 9.94 +/-2.41*** | 11.26 +/-2.64 |
| AP u/L | 52.8 +/-6.7 | 63.8 +/-16.9 | 82.3 +/-14.8 | 64.3 +/-19.6 | 58.3 +/-7.6 | 64.8 +/-8.8 |
| TBIL umol/L | 5.81 +/-0.17 | 3.93 +/-0.51 | 3.42 +/-0.00**** | 2.56 +/-0.51**** | 2.22 +/-1.03 | 1.71 +/-1.03* |
| CPK u/L | 22.3 +/-1.9 | 19.3 +/-5.3 | 27.3 +/-4.8 | 57.8 +/-35.0 | 22.8 +/-5.7 | 223.0 +/-152.4*** |
| CARDIOVASCULAR PARAMETERS | ||||||
| CONTROL | 1 | 2 | 3 | 4 | 5 | |
| HR b/min | 102 +/-9 | 135 +/-16* | 128 +/-15 | 128 +/-16 | 111 +/-10 | 78 +/-3 |
| AP mmHg | 94.5 +/-1.9 | 111.8 +/-2.1*** | 122.4 +/-4.2** | 122.8 +/-5.6* | 135.5 +/-6.0** | 134.3 +/-7.4* |
| PA mmHg | 12.5 +/-0.7 | 12.6 +/-1.3 | 16.2 +/-1.2* | 18.8 +/-2.8* | 20.5 +/-1.2*** | 16.5 +/-1.4 |
| LA mmHg | 4.7 n=3 | 5.0 n=2 | 4.5 n=2 | 5.0 n=2 | 4.5 n=2 | 9.5 n=2 |
| AF L/min | 3.5 +/-0.2 | 3.0 +/-0.4 | 2.7 +/-0.2* | 2.6 +/-0.5 | 3.2 +/-0.3 | 3.0 +/-0.8 |
| CO mL/min/kg | 123 +/-9 | 105 +/-17 | 93 +/-11* | 93 +/-22 | 112 +/-13 | 107 +/-34 |
| SV mL/kg | 1.23 +/-0.09 | 0.85 +/- 0.22 | 0.78 +/-0.15* | 0.86 +/-0.36 | 1.07 +/-0.24 | 0.33 +/-0.37 |
| SVR arbitrary units | 0.80 +/-0.07 | 1.15 +/-0.14* | 1.39 +/-0.16* | 1.56 +/-0.36 | 1.26 +/-0.14* | 1.65 +/-0.43 |
| PDP mmHg | 7.3 n=3 | 9.5 n=2 | 10.5 n=2 | 9.5 n=2 | 14.0 n=2 | 6.0 n=2 |
| PVR arbitrary units | 0.06 n=3 | 0.10 n=2 | 0.12 n=2 | 0.12 n=2 | 0.15 n=2 | 0.10 n=2 |
| dP/dT:llT /sec^2 | 2950 +/-190 | 2860 +/-190 | 2700 +/-280 | 2200 +/-480 | 2510 +/-130 | 3060 +/-300 |
| PVO2 mmHg | 38.1 +/-1.0 | 41.3 +/-0.8* | 40.7 +/-1.3 | 41.0 +/-1.8 | 41.5 +/-1.2* | 39.4 +/-0.5 |
| PVCO2 mmHg | 37.1 +/-2.1 | 36.4 +/-2.1 | 36.9 +/-2.6 | 40.5 +/-2.5 | 40.0 +/3.1 | 47.0 +/-2.2*** |
| pHV | 7.385 +/-0.004 | 7.397 +/-0.012 | 7.372 +/-0.014 | 7.364 +/-0.005 | 7.316 +/-0.011** | 7.295 +/-0.016** |
| heart rhythm abnormalities n=7 | vent tachy(dog#1) normal (#2) sinus tachy (#6) |
sinus arrhyth with episodic vent beats (#1) sinus tachy (#6) sinus tachy (#7) |
normal (#1) sinus tachy (#2) sinus arrest (#4) sinus tachy (#6) PVC progressing to multifocal vent tachy (#6) |
sinus brady, sinus arrest, occas vent
escaoe beats (#1) normal (#2) normal (#4) vent tachy (#5) sinus tachy (#6) normal with wandering pacemaker progressing to vent tachy (#7) sinus arrest (#8?) |
sinus brady (#2) sinus brady, sinus arrest (#4) AV dissociation with synchrony then atrial standstill with vent ectopic beats (#5) |
|
| Configuration abnormalitites | T wave amplitude increased (#2) | S-T segment depression (#1) | S-T segment depression (#1) T wave amplitude increased (#2) Overall amplitude decreased(#4) |
biphasic T waves (#2) mild Q-T segment prolongation (#4) T wave amplitude increased (#6) Overall amplitude decreased (#8?) |
T wave amplitude increased, R wave
disappearance (#2) T wave amplitude increased, mild Q-T prolongation (#4) |
|
| heart rate beats/min + PaO2 mmHg | 70-160 + 80-106 (normal range) | 160 + 106 (#1) 110 + 92 (#2) 170 + 94 (#6) |
110 +84 (#1) 170 + 86 (#6) 165 + 67 (#7) |
100 + 95 (#1) 165 + 92 (#2) 100 + 84 (#4) 170 + 76 (#6) + 68 (#6?) |
60 + 70 (#1) 130 +72 (#2) 110 + 72 (#4) 160 + 68 (#5) 165 + 47 (#6) 70 +67 (#7) 80 + 94 (#8?) |
50 + 25 (#2) 50 +33 (#4) 80 +19 #5) |
| HAEMATOLOGY | ||||||
| CONTROL | 1 | 2 | 3 | 4 | 5 | |
| PCV L/L | 0.46 +/-0.01 n=10 | 0.50 +/-0.02 n=5 | ||||
| TPP g/L | 67.8 +/-1.1 n=10 | 75.8 +/-4.3 n=5 | ||||
| Hb g/L | 147.1 +/-3.4 n=10 | 166.8 +/-7.7** n=5 | ||||
| RBC x10^12/L | 6.36 +/-0.16 n=10 | 6.80 +/-0.30 n=5 | ||||
| WBC x10^9/L | 15.38 +/-1.15 n=10 | 20.82 +/-4.84 n=5 | ||||
| ABBREVIATIONS | ||||||
| pHa = arterial pH AF = mean aortic flow ALB = albumin AP = mean arterial pressure AP = alkaline phosphatase Ca = calcium CHOL = cholesterol CO = cardiac output CPK = creatine phosphokinase dP/dt:llT = myocardial contracility f = respiratory rate GLUC = glucose Hb = haemoglobin K = potasium LA = mean left atrial pressure Mg = magnesium Na = sodium PA = mean pulmonary artery pressure PaCO2 = arterial carbon dioxide PaO2 = arterial oxygen tension PAO2-PaO2 = alveolar -arterial oxygen tension difference PCV = packed cell volume PDP = pulmonary driving pressure pHv = mixed venous pH PO4 = phosphate PvCO2 = mixed venous CO2 PvO2 = mixed venous oxygen tension PVP = pulmonary vascular resistance RBC = total red blood cell count Std HCO3 = standard bicarbonate SV = stroke volume SVR = systemic vascular resistance TBIL = total bilirubin TCO2 = total carbon dioxide tE = expiratory time TPP = total plasma protein U = urea VE = expiratory volume V'E = expiratory minute volume WBC = total white blood cell count |
||||||
References
Atwell R and Fitzgerald M (1994) Unsolved issues in tick paralysis. Australian Veterinary Practitioner, 24(3) 156-161.
Collins, Henry (1997) Paralysis Tick- the plot thickens; Boehrniger Ingelheim Newslletter, Aug 1997 No 33.
Ilkiw JE (1983) Tick Paralysis in Australia, in Current Veterinary Therapy IX, ed RW Kirk, Lea and Febiger.
Ilkiw JE and Turner DM and Howlett CR (1987) Infestation on the dog by the paralysis tick Ixodes holocyclus 1. Clinical and histological findings. Australian Veterinary Journal 64 (5) 137-139.
Ilkiw JE and Turner DM CR (1987a) Infestation on the dog by the paralysis tick Ixodes holocyclus 2 Blood gas and pH, haematological and biochemical findings. Australian Veterinary Journal 64 (5) 139-141.
Ilkiw JE and Turner DM CR (1987b) Infestation on the dog by the paralysis tick Ixodes holocyclus 3 Respiratory Effects. Australian Veterinary Journal 64 (5) 142-144
Ilkiw JE and Turner DM CR (1988) Infestation on the dog by the paralysis tick Ixodes holocyclus 4 Cardiovascular Effects. Australian Veterinary Journal 65 (8) 232-235.
Ilkiw JE and Turner DM CR (1988) Infestation on the dog by the paralysis tick Ixodes holocyclus 5 Treatment. Australian Veterinary Journal 65 (8) 236-338.
Jones, D K (1991) Tick Paralysis; in Emergency Medicine and Critical Care; Proceedings 149, Post Graduate Committee in Veterinary Science, University of Sydney.
Malik R, Farrow, BRH: Tick Paralysis in North America and Australia, in The Veterinary Clinics of North America, Small Animal Practice, Vol 21:1 Tick Transmitted Diseases, 1991.
Malik R, King J and Allan GS (1988) Megaoesophagus associated with tick paralysis in three dogs. Australian Veterinary Practitioner 18(4) 156-159
The Paralysis Tick of Australia - Home
E-mail Us to report a broken link!