TICK-TRANSMITTED DISEASES in HUMANS (world-wide)
When a tick feeds it anchors its mouthparts deep within the skin and injects saliva. Excess water extracted from the ingested blood is voided back into the host through the salivary glands. Hence ticks, especially the nymph and adult stages, are efficient transmitters of pathogens, namely viruses, bacteria and protozoa. With respect to Ixodes holocyclus there is however still much to be learnt about potential vector competence.
For an information kit and a contact list concerning Australian tick-transmitted diseases contact Tick Action Group Support (TAGS) by writing to TAGS at PO Box 95, Mona Vale, NSW 1660.
Viruses
Arboviruses
- Russian spring-summer encephalitis(RSSE)
- Tick-borne Encephalitis (TBE)
- Omsk haemorrhagic fever (OHF)
- Kyasanur Forest disease (KFD)
- Crimean-Congo haemorrhagic fever (CCHF)
- Colorado tick fever (CTF)
- Miscellaneous arboviruses
Bacteria
Rickettsiae (see definition)
- Coxiella burnetii (Q Fever, World Wide, mostly contracted from infected livestock rather than ticks)
- Rickettsia australis (Rickettsial Spotted Fever, Australian Spotted Fever, Queensland Tick Typhus, Australia)
- Rickettsia honei (Flinders Island Spotted Fever, Tasmania, Victoria, Flinders Is, Australia)
- Rickettsia rickettsii (Rocky Mountains Spotted Fever, USA)
- Rickettsia conori (Boutonneuse fever, Africa & Mediterranean )
- Ehrlichia sp. (USA)
Spirochaetes
- Borrelia burgdorferi (Lyme disease)
- ? Australian Borrelia sp?
Other bacteria
- Francisella tularensis (Tularaemia) (from rabbit ticks)
Protozoa
- Babesia sp. (USA)
Arboviruses
Arbovirus- "Any one of over 200 arthropod-borne viruses biologically transmitted between susceptible vertebrate hosts by haematophagous (blood sucking) arthropods". More than 100 arboviruses have been recovered from ticks, but all the important tick-borne viral diseases are spread by hard ticks. Most are encephalitides, i.e. they produce clinical responses in which encephalitis is the predominant feature, only a few viruses such as Colorado tick fever produce generalised infections. All arboviruses are transmitted by the tick's bite, and transovarial transmission usually occurs.
In mainland Australia, no viruses responsible for any known human infections have been isolated from ticks. [Note that this does not exclude Rickettsial Spotted Fever, Q Fever and Lyme-like Australian borreliosis which are all bacterial rather than viral diseases]. There have, however, been instances of various viruses causing human illness being isolated from ticks on seabirds in the Great Barrier Reef and Tasmania (Russell and Doggett, 1995).
Russian spring-summer encephalitis (RSSE): taiga forests of former USSR, Siberia, Asia, China; Ixodes persulcatus, Haemaphysalis concinna.
Tick-borne Encephalitis (Central Europe, TBE): Scandinavia to the Balkans, Ixodes ricinus, Dermacentor marginatus, Haemaphysalis spp.
Omsk haemorrhagic fever (OHF): Western Siberia, Dermacentor and Ixodes spp.
Kyasanur Forest disease (KFD): Southern India, Haemaphysalis spinigera.
Crimean-Congo haemorrhagic fever (CCHF): Crimea, France, Portugal, Bulgaria, Egypt to South Africa, Mauritania to Kenya, Middle East, Pakistan, India and China. Enzootic in savannah, steppe and semidesert areas; Hyalomma marginatum, Dermacentor marginatus, Amblyomma, Rhipicephalus, Boophilus, Hemaphysalis spp.
Colorado tick fever (CTF): Rocky Mtn States and S Dakota and Western Canada; Dermacentor andersoni, Haemaphysalis, Otobius lagophilus. Trans-stadial transmission.
Miscellaneous arboviruses: Powassan encephalitis (N America, former USSR), Langat virus (Malaysia), Louping ill (Britain)
Rickettsiae
Q Fever: has been recognised since 1946 as a source for infection with the organism Coxiella burnetii; has a world-wide distribution and is mainly a disease of rodents and other mammals. It is mainly spread by consuming contaminated milk and meat from cattle, and the inhalation of dried infected faeces by those working with sheep, cattle and goats. Hard ticks (and also soft ticks) may help maintain an enzootic cycle, and even spread the infection to humans by their bites or infected faeces. Transovarial transmission occurs. Whilst Q Fever is often quoted as being transmitted by ticks, there is very little information to support this, especially as the causative organism is readily passed on via aerosols (abattoir workers are at the greatest risk).
Australian Rickettsial Spotted Fever(s):
In general the vectors of spotted fevers are Ixodid ticks, and quite frequently it is their larval stages. Trans-stadial and trans-ovarial transmission occurs. Normal hosts are bandicoots and, to a lesser extent, brushtail possums. There is now thought to be more than one kind of spotted fever in Australia.
- Rickettsia australis (Spotted Fever, Rickettsial Spotted Fever, Queensland Spotted Fever, Tick Typhus, Queensland Tick Typhus [QTT])
- Rickettsia honei (Flinders Island Spotted Fever [FISF])
Rickettsia australis. An eastern Spotted Fever. In Queensland, the disease is called "Queensland Tick Typhus (QTT)". It is a tick-borne infection with symptoms easily confused with Ross River virus disease. It was first called "North Queensland Tick Typhus" but the main focus of the disease was later found to be in the southeast of the state. "Tick typhus", as it was later colloquially known, emerged as a problem during World War II among troops training for jungle warfare. No cases were detected south of Lismore until the late 1970's. Then in the space of 7 mths a little girl and a man were infected after tickbites in the northern suburbs of Sydney. It appears that this organism must be considered a possibility throughout the east coast range of the paralysis tick. So far however there seems to be no evidence for spotted fevers occurring along the south coast of New South Wales, but this is probably because no one has looked. PCR is now being utilised in Victoria for laboratory confirmation of the disease. In NSW in 1997 there were less than 50 cases of QTT reported annually. In 2000, the condition is being frequently diagnosed in the densely populated northern beaches suburbs of Sydney. In southern states there are possibly other rickettsial species that remain to be identified. Tick Action Group Support (TAGS) provides an information kit on Australian tick-transmitted diseases by writing to: TAGS at PO Box 95, Mona Vale, NSW 1660. Also see Westmead Hospital's page on Spotted Fevers for updates.
Rickettsia honei. Commonly known as Flinders Island Spotted Fever (FISF) Rickettsia honei infection occurs in eastern Victoria and Tasmania, as well as on Flinders Island. Whilst it appears that the spotted fevers are transmitted to humans by the Paralysis tick, Ixodes holocyclus, other species such as I. tasmani are also thought to be involved in the transmission of this infection.
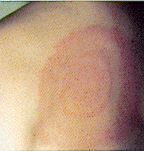
Clinical Signs of Australian Spotted Fevers.
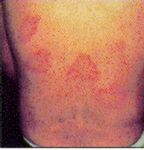
Usually the patient is unaware he or she has been bitten until illness occurs. In 65% of cases there is an eschar (a black spot) 2-5 mm in diameter at the site of the tick bite. This looks like a scab with redness and swelling around it. Incubation period is 7 to 10 days and initial symptoms include headache, fever, muscle pain, joint pain, stiff neck, nausea, vomiting, and mental confusion. There may be just a high fever. Often local lymph nodes are enlarged and sore. Fever starts 1-14 days following tick bite, followed within a few days by a rash (usually 3-5 days, but can be 1-14 days after onset of symptoms) . This rash can be red spots (erythematous macules) or maculopapular (raised spots) and it develops on the trunk, and later the face, palms and soles. It can look like chicken pox and the spots may contain some fluid. Sometimes there is no rash at all. The disease is apparently not uncommonly seen in tick collectors in southern Queensland. It may be more severe in adults and elderly people. One bout of the disease seems to confer solid immunity. It is rarely fatal.
Diagnostic serology is available in the form of IgM and Weil-Felix tests. Two blood tests are taken at least 10 days apart. The disease runs it's course in two weeks or so but can be cured more quickly with antibiotics. Changes in the regional lymph nodes draining the area of attachment can also lead to the formation of chronically sensitive enlargement within the node lasting for several years, particularly those around the head and neck. Treatment is with tetracyclines and is usually rapidly effective, although rare fatalities have been recorded.
[Not all human cases seem to follow such a benign course. One person has emailed (to the web author) to say that she had an initial typical bout of fevers and rash lasting 3 weeks which was treated with tetracyclines for 1 month. She had an apparent relapse 6 months later and has now had the main tick lesion cut out. A biopsy of the site 6 years after the initial attack revealed the continued presence of the bacteria (presumably R. australis). Since the excision she has had two recurrences of the disease's rash (between the shoulder blades and the right belt line) although not nearly as severe as the original attack. She has since also had illness from arthritis and hepatopathy.]
Abstract of article on Rickettsial Spotted Fever
Author: Sexton DJ; Title: Spotted fever group rickettsial infections in Australia. Dwyer BDivision of Infectious ; Kemp R; Graves S. Address: DiseasesDurham, North , Duke University Medical Center, Carolina Sep-Oct, 13:5, 27710. Source: Rev Infect Dis, 1991 876 -86. Abstract: More than four decades ago, Rickettsia australis Queensland was discovered to be the etiologic agent of tick typhus persist about (QTT), yet many unanswered questions the ecology, epidemiology, and clinical features of this disease. We review previously published cases of QTT along with 16 46 cases discovered by active surveillance. Patients often have regional lymphadenopathy. QTT is usually a mild diseaseand escharsvesicular rashes. Because clinical features overlap, . Some have serologic tests are necessary to distinguish QTT from other endemic Australian rickettsial diseases (vectors of R. australis have been identified: Ixodes holocyclus and Ixodes tasmaniscrub and murine typhus). Only two tick rickettsiae are isolated from patients in Victoria and . Until Tasmaniafever group infections in these locations are due to R. australis, it remains unproven that spotted epidemiologic, and clinical data suggest that QTT is not . However, available serologic, confined to the area in which was first isolated (Queensland); rather, it occurs along a 200-km span of eastern coastal AustraliaR. australis 3, from tropical to temperate climates. ,
Rocky Mountain spotted fever: Rocky Mounted Spotted Fever - this had a fatality rate of around 30% before the advent of antibiotics. Caused by Rickettsia rickettsii. There is sudden onset of headache, chills and fever; a characteristic exanthem (skin eruption) occurs on the extremities and trunk.
Siberian tick typhus/ North Asian tick typhus:
Boutonneuse fever: The prototype of the tick-borne typhus fevers of Africa, widely distributed in Africa and the Mediterranean, Caspian and Black Sea basins. Caused by Rickettsia conori and transmitted by the bite of Ixodid ticks, with dogs and rodents as animal hosts, Clinically characterised by a necrotic initial lesion (tache noire), headache, fever, rash, and generally a favourable prognosis.
Ehrlichiosis. (Taken from The Wall Street Journal - 8/17/95) A new hazard is joining sand traps and ponds on the nation's golf courses. The hazard is a little-known disease borne by ticks, and it threatens not only golfers but almost anyone else who ventures into the woods, especially in the South. The disease is the second obscure tick-borne illness to grab the attention of epidemic experts in the last several months. Researchers at Vanderbilt University and the Centers for Disease Control and Prevention reported that they analyzed a 1993 outbreak of a tick-borne disease call human erlichiosis at a retirement community in Tennessee with several golf courses. Golfers who searched for errant balls off the fairway into the woods were almost four times more likely to show evidence of past infection with the potentially fatal disease than those who played new balls. Perhaps more seriously, the same scientists reported that the little-known disease may be more common than previously realized. Only about 400 cases of the flu-like erlichiosis have been confirmed nation-wide since the ailment was identified in 1987. But 12.5% of the 3,000 retirees in the Tennessee comminity showed evidence of previous infection by the erlichiosis bacterium, Vanderbilt University's William Schaffner and his colleagues report in this week's New England Journal of Medicine. Eleven of these residents developed full-blown cases of the disease, and several required long hospital stays. This rate of illness was 200 times higher than in previous studies, Dr. Schaffner's team reports. "My guess is that this is more common than Lyme disease in the southern U.S.," said Sam Telford, a parisitologist at the Harvard School of Public Health. Cases also have been reported in the Northeast and Midwest. The ailment is carried by the Lone Star tick, one of the more common ticks in the Southeast. Erlichiosis is easy to misdiagnose, because its initial symptoms - fever, headache, and nausea - resemble many other maladies, including Lyme disease. Unlike Lyme disease, though, it almost never causes skin rash. If untreated, the disease often rapidly worsens, and can cause kidney and respiratory failure. Moreover, the ailment responds to only one of several antibiotics that are used for treating Lyme disease - so a misdiagnosis could have serious consequences. Human erlichiosis isn't the only new tick scourge golfers and hikers have to worry about. A sister disease, human granulocytic erlichiosis, was identified last year (1994). It garnered headlines in recent months after causeing outbreaks - and a few deaths - in the upper Midwest and New York. Just this week, Harvard's Dr. Telford and his colleagues verified the first case in Massachusetts. HGE is carried by the same deer tick that causes Lyme disease. The specialists suggested that people who most go into the woods these days use insect repellent and check for ticks afterward. "It's a wonderfully comforting fact. Insect repellents work," said Dr. Schaffner, chief of preventive medicine at Vanderbilt's medical school.
Spirochaetes
Whilst the North American Lyme Disease has not actually been confirmed in Australia by culture of the organism (see Westmead Hospital's Dept of Medical Entomology- tick facts sheet), the presence of a similar clinical syndrome combined with positive antibody tests suggests that a similar spirochaete infection from ticks does occur, or at least similar to the European form.
Lyme Disease is caused by a group of microscopic spiral bacteria (spirochaetes) transmitted to humas and animals through the bite of a tick, often very small in size. In North America the bacterial species is Borrelia burgdorferi, in Europe it is Borrelia afzelii and Borrelia garinii. Whilst the causative species in Australia is unknown (2000), the disease syndrome seen here is more similar to the European form than the North American one. Attempts to isolate the organism from ticks and from biopsied skin lesions are continuing at Royal North Shore Hospital's Tick-Borne Diseases Research Unit under Dr Bernie Hudson (infectious diseases specialist and microbiologist). General Practitioners (medical) can contact the Secretary of the Microbiology Department at Royal North Shore Hospital for information on how to participate in the study (ph 02 9926 8480).
In the US, Lyme disease is a big issue with around 10,000 new cases every year, most in the North East of the country (but 43 States have reported it). The classic form of the disease was first described in the town of Lyme, USA in 1975, and isolated in 1981. The disease has also been reported in at least 20 countries, across six continents around the world. In Australia, the disease has been reported since 1982 and is found mainly in areas on the Eastern seaboard. However isolation of virulent Borrelia burgdorferi s.l. from Australian Ixodid ticks has not yet been achieved. The common native paralysis tick (bush tick, scrub tick, Ixodes holocyclus), in all its stages (larva, nymph and adult) is thought to be the transmitter of the bacteria.
Incubation period is thought to range from 3 to 30 days after a bite from an infected tick. Often this lengthy elapsed time causes considerable confusion for diagnosticians. The clinical signs of human borreliosis in Australia are similar but not quite the same as the classic North American one.
A wide variety of tissues may be invaded, and may lead to permanent injury to joints, heart, central nervous system and other internal organs. Early recognition and treatment with antibiotics over several weeks can halt the disease, but delay may result in a lifetime of chronic illness and fatigue. There are a wide variety of symptoms,such as headache, stiff neck, fever, flu-like symptoms, muscle ache, fatigue or vomiting. A rash may develop within hours or days after the tick bite but is not always present. (see Clinical Features of North American Lyme Disease below) .
Differential diagnosis might include Dengue fever, Malaria,
Glandular Fever, Rickettsial Spotted Fever, Epidemic Poly-arthritis
and Rheumatoid arthritis. Diseases similar to Lyme disease caused
by related borrelia and transmitted by Ixodid ticks also occur in
Europe and Japan. As yet there is no definitive test for
Borreliosis in Australia because the organisms are difficult to
culture and hence to identify and characterise. The Tick Diseases
Research Unit (TDRU) at the Royal North Shore Hospital in Sydney,
comprising specialist physicians, parasitologists,
microbiologists, medical practitioners and other health workers
is investigating tick-borne infections in the Sydney region. See
also Vector Competence of Ixodes
holocyclus- Lyme Disease in Australia?. It is not known
whether "Australian borreliosis" affects domestic
animals but some animals with tick paralysis have apparently
subsequently developed signs of polyarthritis, CNS disturbances
and heart conditions (Collins, 1997).
See also Tick-transmitted Diseases Australia (on
this website).
Other bacteria
Tularaemia. 1. Agent: Francisella tularensis (gram-negative and aerobic). 2. Epizootiology: Although numerous species, including laboratory rabbits, are susceptible, from a practical standpoint the disease is important mostly as a zoonotic hazard associated with wild rabbits (Sylvilagus spp.) and hares (Lepus spp.). Direct contact and wound entry or penetration of intact skin are most common means of transmission to man, but transmission by inhalation or ingestion also occurs. Among wild animals, infection is spread primarily by blood-sucking or biting arthropods, which may be merely mechanical or true biological vectors. Human infections also have resulted from tick bites. 3. Clinical: Affected animals usually are found dead; signs when observed are nonspecific. In man, fever and lymphadenopathy with or without abscess or phlegmon at initial site. Can be fatal if untreated, but responds well to antibiotics. 4. Pathology: Multifocal necrotizing and suppurative lesions in many organs: liver, spleen, lungs, lymph nodes, bone marrow, et al. 5. Diagnosis: Lesions, culture. 6. Control: Prevent vectors from entering colony. Use care when dressing wild rabbits. 7. References: Evans ME et al. 1985. Tularemia: A 30-year experience with 88 cases. Medicine (Baltimore) 64:251-269. Gordon JR et al. 1983. Tularemia transmitted by ticks (Dermacentor andersoni) in Saskatchewan. Can J Comp Med 47:408-411. Morner T et al. 1988. Infections with Francisella tularensis biovar palaearctica in hares (Lepus timidus, Lepus europaeus) from Sweden. J Wildl Dis 24:422-433. Rohrbach BW. 1988. Zoonosis update. Tularemia. J Am Vet Med Assoc 193:428-432.
Protozoa
Babesiosis.United States' West Coast residents aren't off the hook, either. Early in 1995, another team of researchers verified that four Califormia residents had been infected with a new version of tick-borne babesiosis, a malarialike malady. Until recently, babesiosis was thought to reside only in the eastern part of the country. Babesiosis is a febrile haemolytic disease, primarily observed in animals and rarely in man, caused by infection with Babesia. Babesia is a genus of intracellular, non-pigmented sporozoa which invade the red blood cells of cattle, sheep, horses, rodents, dogs and monkeys, but rarely of man. Members of this genus are oval or pear shaped.
Note
In Borrelia burgdorferi s.l. the "s.l." is an abbreviation for senso lato meaning literally in the wide sense. This is because there are now many recognised spirochaetes that used to be all called "B.burgdorferi". To name a few: B.garinii, B.afzelii, B.valaisiana, B.louisitania, B.japonica, plus undoubtably more to be discovered. Researchers at Royal North Shore hospital suspect that any Australian borreliosis is a disease more akin to the European Lyme disease, such as that caused by B.garinii (S Doggett pers. com.).
Bibliography
Collins, Henry; Senior Lecturer in Veterinary Parasitology, Dept of Veterinary Anatomy and Pathology, University of Sydney; Boehringer Ingelheim Newsletter August 1997 No 33
McManus, T J: Wildlife Zoonoses in Proceedings No Australian Wildlife; University of Sydney Post- Graduate Committee in Veterinary Science, 19.
Service, MW: Medical Entomology For Students: Chapman and Hall, London, 1996.
Lyme Disease- A Monograph and Guide for Washington Physicians
| Clinical Features The clinical
manifestations of Lyme disease are variable from person
to person. Parallels have been drawn between Lyme disease
and syphilis because both are caused by spirochetes, both
can involve multiple organ systems at different stages of
the infection, and both can mimic other diseases. Common
clinical manifestations and typical time courses of the
different stages of Lyme disease are described in this
monograph. Conceptually, it is useful to think of Lyme
disease in three different stages (Table 1).
Alternatively, one can consider stages 1 and 2 as early
Lyme disease, and stage 3 as late Lyme disease.
Although the clinical manifestations have been divided into stages, each person may have a unique presentation and progression of symptoms.
|
| Table 1 Major Manifestations of Lyme Disease* |
|||
| System | Early | Late | |
| Stage 1 Localized |
Stage 2 Disseminated |
Stage 3 Chronic |
|
| Skin | Erythema migrans | Secondary annular lesions | Acrodermatitis chronica atrophicans (Europe) |
| Musculo- skeletal |
Myalgias | Migratory pain in joints, bone, muscle; brief arthritis attacks | Prolonged arthritis attacks, chronic arthritis |
| Neurologic | Headache | Meningitis, Bell's palsy, cranial neuritis, radiculoneuritis |
Encephalopathy, polyneuropathy, leukoencephalitis |
| Cardiac | Atrioventricular block, myopericarditis, pancarditis | ||
| Constitutional | Flu-like symptoms | Malaise, fatigue | Fatigue |
| Lymphatic | Regional lymphadenopathy | Regional/generalized lymphadenopathy | |
| *This table was adapted with permission from: Steere AC. Lyme Disease. N Eng J Med 1989; 321:589 | |||
This monograph was produced for the World Wide Web by the Northwest Center for Public Health Practice in cooperation with the Washington State Department of Health. It was adapted from the original print version. See bibliography for acknowledgements.
E-mail Us to report a broken link!